SHARE
Adalimumab (brand name Humira®) is one of the most common “biologic” medications that we use for patients suffering from Rheumatoid arthritis. This is a powerful medication that we use for patients that failed other therapies like disease modifying anti-rheumatic drugs (DMARDs) or non-steroidal inflammatory medications. Education is key in understanding the benefits but also the risk of using this medication.
Thus, I discuss with my patients in detail and here are the most common questions that I answer for my patients.
- What is Adalimumab/ Humira?
- What is the effect of Humira in Rheumatoid arthritis patients?
- Is Humira a pill or an injectable medication?
- What tests are needed before starting Humira?
- Can Humira be combined with other medications such as biologics?
- What are the most common side effects?
- Can Humira increase the risk of infections and how common are the SEVERE infections?
- How can I decrease and prevent infections?
- Can Humira increase the risk of cancer?
- Is Humira safe for patients with heart failure?
- What vaccines are recommended before starting Humira?
- Is Humira safe for pregnancy?
What is Adalimumab/ Humira?
Adalimumab, sold under the brand name Humira, is a “biologic” medication from the TNF-alpha inhibitors class. It is prescribed to patients with moderate to severe cases of Rheumatoid arthritis. Humira is a recombinant monoclonal antibody. It binds to human tumor necrosis factor alpha (TNF-alpha), a well-known molecule that causes inflammation in our body.
What is the effect of Humira on Rheumatoid arthritis?
Patients with Rheumatoid arthritis have high levels of inflammation. Inflammation may be present systemic (affecting many organs) or in the joints. Treatment of Rheumatoid arthritis with Adalimumab (Humira) works to reduce signs/ symptoms and inhibit progression of structural joint damage, but also improve physical function. Humira interferes with TNF-alpha molecules, which can be found in high levels in the synovial fluid of patients suffering from Rheumatoid arthritis.
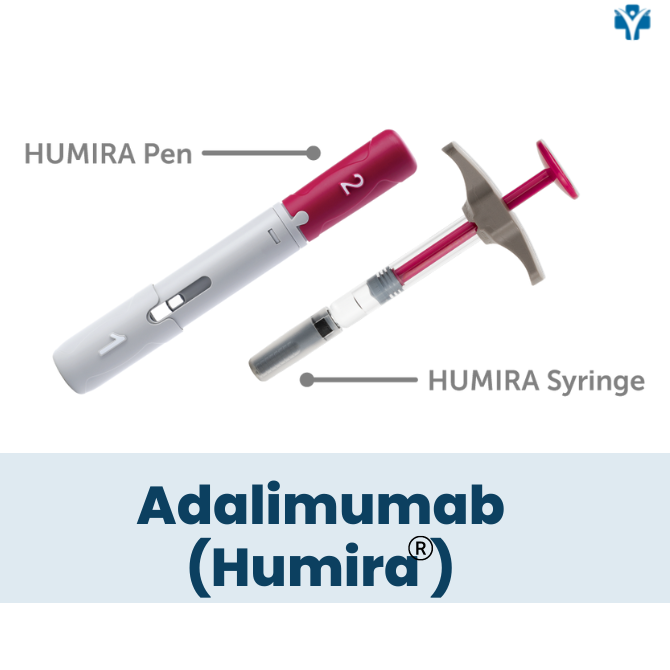
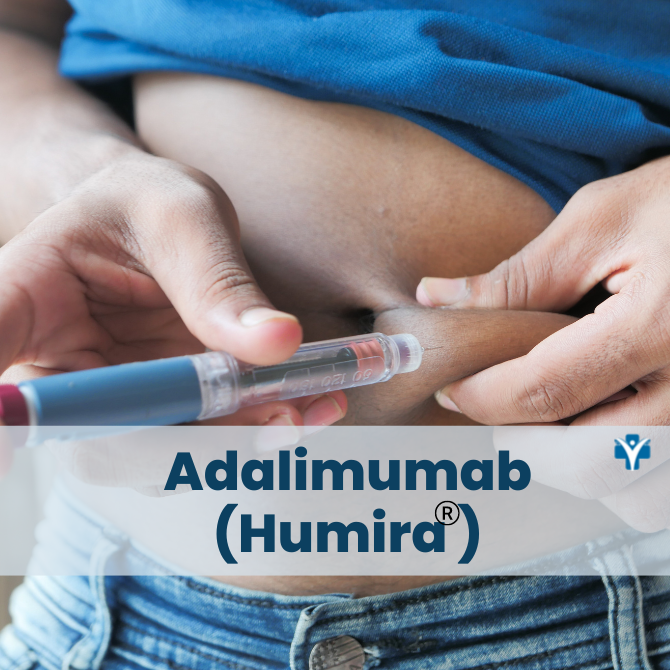
Is Humira a pill or an injectable medication?
Humira is a self-administered injectable medication usually taken by a 40 mg injectable pen or syringe every other week. It may be given in the thigh or abdomen area. It is recommended that you rotate the site of the injection whenever you give yourself the injection. You should leave it at room temperature for about 15- 30 minutes before use, but do not remove the cap or cover during this time. Do not use it if the solution is discolored. Do not administer on areas of skin that are red, bruised, hard, or have scars.
What tests are needed before starting Humira?
Before starting therapy with Adalimumab (Humira), it is important to screen for latent tuberculosis and hepatitis B and , or hepatitis C. You will also need other tests like: CBC with differential, complete metabolic panel, and HIV screening in high risk patients. Laboratory tests are also needed during therapy with Humira. Discuss with your physician about how frequent you need testing.
Can Humira be combined with other medications, such as biologics?
Adalimumab (Humira) is safe to be combined with other disease-modifying anti-rheumatic drugs (DMARDs; e.g methotrexate, leflunomide, sulfasalazine, and hydroxychloroquine). However, it is not recommended to be associated with other TNF- alpha inhibitors or biologics due to the increased risk of infections.
What are the most common side effects Humira?
Here are listed the most common side effects reported by patients on Adalimumab (Humira):
- Skin rash
- Fever
- Positive ANA titer
- Antibody development
- Infections (upper respiratory infections such as sinusitis)
- Injection site reaction
- Nervous system symptoms such as headache and torso pain
- Cardiovascular complications such as acute myocardial infarction, cardiac arrhythmia, chest pain, and palpitations
- High cholesterol
- Gastrointestinal issues such as abdominal pain
- Hypersensitivity reaction
- Back pain
- Alopecia
Can Humira increase the risk of infections?
Humira puts patients at an increased risk of developing serious infections, mainly if used in combination with other drugs that lower your immune system (e.g, methotrexate; corticosteroids). The risk is about 4-5%.
Infections include active tuberculosis, the reactivation of latent tuberculosis, invasive fungal infections, or other bacterial and viral infections (e.g herpes zoster, hepatitis B). If you get an infection, you should stop the medication and call your physician immediately.
How can I decrease and prevent infections?
I recommend getting up to date with all immunizations (e.g. flu vaccine, pneumococcal vaccine, COVID-19 vaccine, Herpes Zoster vaccine) to all my patients before starting therapy unless they have contraindications. However, I recommend against getting any LIVE vaccines during therapy with Humira.
Can Humira increase the risk of cancer?
Lymphoma and other malignancies are rare, but they have been reported in patients taking Humira ( e.g. lymphoma and nonmelanoma skin cancers). However, the risk of getting a malignancy on this medication is still under debate.
Is Humira safe for patients with heart failure?
Patients on TNF- alpha inhibitors rarely may develop new-onset or worsen of heart failure. Thus, if you suffer from a cardiac disease, disclose this to your physician, as you will need a complete cardiac evaluation before starting this therapy.
What vaccines are recommended before starting Humira?
Being up to date with all immunizations is recommended before starting therapy with Humira. Live vaccines should not be given concurrently with Humira.
Inactivated vaccines should be given at least 2 weeks before initiation of therapy, and patients vaccinated less than 14 days before initiating or during therapy should be revaccinated at least 2-3 months after treatment is complete.
Is Humira safe for pregnancy?
The risk of using Adalimumab/ Humira during pregnancy is moderate to low. No risks of adverse maternal or fetal effects have been observed following Adalimumab exposure during pregnancy.
If you remain pregnant on this medication, contact your physician. It is considered safe to continue therapy until 32 weeks of pregnancy. TNF-alpha blockers are considered safe with breastfeeding.
This medication information is limited. Patients should use it as a tool better to understand the medication’s role in disease treatment. It is not supposed to be comprehensive and does NOT include all information about a diagnosis, treatment, medication, side effects, or risks that may apply to a specific patient. It is not intended to be medical advice or a substitute for a physician’s medical advice, diagnosis, or treatment. Patients must speak with their physician for complete information about their health, medical questions, and treatment options, including any risks or benefits regarding use of medications. This information does not endorse any treatments or medications as safe, effective, or approved for treating a specific patient.